Adelaide-based company TekCyte is now seeking investors to fund the expansion of their revolutionary medtech BIOINVISIBLE – a drug-free medical device coating that could curb global rates of infection from implanted devices and potentially save thousands of lives each year.
Developed for commercialisation out of the Cell Therapy Manufacturing Cooperative Research Centre, based at the University of South Australia, and spearheaded by TekCyte CEO Dr Tony Simula, BIOINVISIBLE is a hydrophilic hyperbranched polyglycerol polymer that acts as a physical barrier on devices such as catheters, orthopaedic implants and stents.
During extensive laboratory testing the remarkable tech demonstrated extreme reduction in biofilm – which means it could drastically reduce infection rates associated with implanted devices. This is in addition to its demonstrated ability to reduce clots, which may reduce blood clots from certain devices like stents and heart valves.
“The World Health Organisation has identified infection as one of their top global concerns due to the growing number of antibiotic resistant bacteria and they anticipate by 2050 there will be 10 million deaths across the planet each year if something isn’t done,” Dr Simula said.
“TekCyte sees a real opportunity with BIOINVISIBLE to tackle the problem of infection from devices like catheters. Virtually every patient admitted to a hospital will have a catheter of some description inserted into their vein. Some catheters like central venous catheters (CVCs) may remain in place for many weeks and, as a result, are much more prone to infection.
“In Australia alone, there are about 4,000 bloodstream infections from CVCs each year and 20 per cent of those patients die. In 2015, this cost Medicare $36 million. In the US, there are around 250,000 infections annually from CVCs with similar death rates.
“And while there are many companies out there that have coatings to prevent biofilm, their tech relies on incorporating drugs, and this all adds to a greater risk of drug resistant bacteria. BIOINVISIBLE, on the other hand, is drug-free and can be chemically bonded to the surface of many devices.”
It’s why Dr Simula is now beelining for the world’s major medtech players, who are based out of the States and Europe, with interest already mounting from companies who produce devices such as catheters.
“Our business model is not to take a BIOINVISIBLE-coated catheter or other device to the market ourselves as that would take up to 10 years for a new company,” Dr Simula said.
“Instead, what we’re aiming to do is to partner with companies that already have a product on the market such as catheters. This reduces time to market as our partner would only need to obtain approval of a new coating on their already approved product. This also gives those companies an opportunity to differentiate from their competitors,” he said.
Dr Simula will travel to Dusseldorf’s Medica Exhibition this November – which is one of the largest medical B2B trade fairs in the world. More than 4500 exhibitors from 66 countries will be on display and an estimated 81,000 visitors from across the globe are expected to attend.
“This is a great opportunity for us to show our tech and gauge more interest in BIOINVISIBLE while showcasing its capabilities,” he said.

Dr Simula has already been working tirelessly with his talented team of eight for years, and commercialisation efforts accelerated once the world opened back up following lengthy COVID-19 lockdowns.
Last year Dr Simula, who founded TekCyte in 2018, after a rich career that spanned publicly listed and private sector biotech/medtech companies, also travelled to New York, Dubai and London for medical conferences where he was able to develop vital networks to pave the way for BIOINVISIBLE’S growth.
He was also asked to present at a Dragon’s Den Innovation Showcase – a program where entrepreneurs pitch their business ideas to a panel of key industry opinion leaders and investors as part of the Charing Cross Vascular Symposium in London.
As to why he thinks the time is now ripe for investors, Dr Simula believes interest is mounting for medtech following a world-wide slump during the pandemic – and with BIOINVISIBLE already showing a range of potential benefits that could extend beyond the medical world, he sees a bright future for this homegrown and life-saving invention.
“There is the potential for BIOINVISIBLE to be used in different ways and we’re already tweaking the chemical process to enable its application to a large number of products such as dental implants and breast implants. We’re also looking at how BIOINVISIBLE can be used to prevent surface fouling – which is the accumulation of unwanted material on solid surfaces. This means it could potentially be used to coat the filtration membranes used in water treatment,” he said.
“We’re super excited by that prospect and it means we have another market that we haven’t even tapped into yet and that’s because we haven’t had the funds and we need investor interest so we can tap into other markets.
“We have already received feedback from US-based surgeons that BIOINVISIBLE could transform the industry and, given the medical coating industry is growing at a ridiculous rate, we feel incredibly optimistic about the impact of our product and its scope.”
Dr Simula said what ultimately makes TekCyte such a special company is the clever team behind him and their unique understanding of the interplay between surfaces and tissue and their ability to change those dynamics for customers.
“Not all coating companies have that capability inhouse. Additionally, our Board are all entrepreneurs in their own right and, from a shareholder perspective, that’s important.”
TekCyte is now welcoming expressions of interest from all investors via their commercialisation partner – the Industry Commercialisation Agency at www.industrycommercialisation.com/tekcyte.
Media contacts:
Sarah Webb | Public Relations Small and Mighty Group
Phone: +61 7 3102 4761 | E: sarah@smallmightygroup.com | W: smallmightygroup.com
Tara James | Managing Director Small and Mighty Group
M: (+61) 0409 330 073 | E: tara@smallmightygroup.com| W: smallmightygroup.com
Learn more about
TekCyte’s next-generation medical device coating technology is helping to create safer and better outcomes for patients. It is anti-thrombogenic, anti-proliferative and evidence also now demonstrates extreme reduction in biofilm.
The BIOINVISIBLE™ globally patented coating technology is an ultra-thin, highly hydrophilic hyperbranched polyglycerol (HPG) polymer that can be chemically bonded to, for example, a stent or catheter surface. This provides a protective barrier against the body’s natural responses to foreign objects, making implantable devices less visible to the body’s immune system.
Stents and other vascular devices coated in this stable and drug-free coating repel accumulation of platelets, proteins, and cells. This aims to reduce complications from stents such as clotting and restenosis and therefore, be more reliable for surgeons and more durable for patients.
“We developed BIOINVISBLE to be easily applied to any existing metal stents. The coating process is scalable to meet future commercial demands, providing medical device manufacturers a reliable, safer and drug-free alternative stent.” says Dr Tony Simula, CEO at TekCyte.
More recently BIOINVISIBLE™ has also been shown to significantly reduce the risk of biofilm on coated surfaces, which could help infection or device failure caused by biofilm. Helping guard against the development of biofilm reduces the likelihood of later medical complications. At the same time, the protective qualities of this world-leading technology also enhance the durability of the medical device that the coating is applied to.
Biofilms are estimated to be responsible for more than 65% of nosocomial infections, almost 80% of chronic infections, and approximately 60% of all human bacterial infections1. Biofilm treatment is very challenging because treatment with antibiotics is often ineffective. Implants are very susceptible to the formation of biofilm, which can develop over days, or even over several months. Once established, surgical intervention to remove/replace the device/implant is the usual course of action.
The safety of any new technology is paramount and according to tests conducted at NAMSA, all studies have successfully shown no evidence of systemic toxicity, irritation, cytotoxicity, hemolysis or sensitivity associated with exposure to BIOINVISIBLE™. This shows the enormous potential of this coating technology as a platform for a range of implanted devices.
“Our research shows that BIOINVISIBLE has the potential to address complications associated with biofilm buildup, without the release of drugs or other active agents,” says Dr Simula.
In fact, TekCyte’s research has shown that catheters and cannulae coated with BIOINVISIBLE could have markedly reduced rates of biofilm from organisms such as Candidaalbicans, Pseudomonas aeruginosa and Staphylococcus aureus.
Urinary tract infections are one of the most common types of infection associated with catheters and is almost always the result of the development of biofilm. According to the Centre for Disease Control in the United States, approximately 75% of UTI’s are associated with a urinary catheter2.
Several studies have shown that opportunistic pathogenic yeast C. albicans can form polymicrobial biofilms, in vitro and in vivo, and that these biofilms can affect disease course and management. Polymicrobial biofilms such as this are often resistant to antimicrobial drugs.

Many researchers have tried to target microbial biofilms to reduce their impact on patient outcomes, however unfortunately current conventional antimicrobial strategies don’t work well to counter biofilm development.
By combating the formation of biofilm on medical devices, it helps to reduce the possibility of later complications for patients. This has long been a complex issue for health practitioners, however, we are now on the cusp of great advances thanks to the active role that BIOINVISIBLE™ can play in reducing complications from medical devices.
“It is clear that BIOINVISIBLE has significant possibilities to address current biofilm challenges and we’re excited by the prospect of working with major device companies to bring to market the first truly biocompatible medical coating” says Dr Simula.
The patented BIOINVISIBLE™ manufacturing process has been scaled up for coating stents and TekCyte is ready to meet commercial demands for the coating in this sector of the industry.
References:
- Candida albicans can form polymicrobial biofilms, in vitro and in vivo https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8894716/#B150
- Assefa M, Amare A. Biofilm-Associated Multi-Drug Resistance in Hospital-Acquired Infections: A Review. Infect Drug Resist. 2022 Aug 31;15:5061-5068. doi: 10.2147/IDR.S379502. PMID: 36068834; PMCID: PMC9441148. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9441148/
Learn more about
The field of biomedical science and technology has been actively working on improving vascular stents to solve problems relating to the body’s reaction to their presence. Advances in nanotechnology are leading to improved vascular stent coating materials, in some cases making the implantable medical device seem invisible to the body. This is helping vascular surgeons to carry out procedures with higher success rates and better outcomes for patients.
What is a Vascular Stent?
A stent is a small metal, wire mesh tube that is placed inside your blood vessel when it is totally or partially blocked. The stent expands the walls of the artery, keeping the blood vessel open so blood can flow through. Stent grafts, however, which are large stents with a polymer fabric lining, can be used to keep weakened or damaged arteries from bursting.
Stents and other intravascular prostheses are used on damaged or diseased vessels to treat a number of medical conditions. While stents are most often used to treat diseases that affect the arteries, such as coronary heart disease or peripheral artery disease, they can also be used to treat blocked veins caused by conditions like deep vein thrombosis (DVT), post-thrombotic syndrome and May-Thurner syndrome.
Why use Vascular Stents?
There are several types of vascular stents all with different purposes to aid a patient’s recovery from a variety of conditions. The common vascular stents are:
Coronary stents aid the arteries leading to the heart. If there is a buildup of plaque in the coronary arteries, it can reduce the blood flow to the heart, leading to blood clots, and more severe heart attacks.
Carotid artery stents help treat carotid artery disease by opening the arteries. Carotid arteries are in the neck and supply blood to the brain. With damage or plaque-blocked arteries, there is an increased risk of stroke.
The arteries in your arms and leg ensure good blood flow throughout the extremities of your body. Peripheral artery disease can lead to buildup of plaque or blood clots in these vessels causing pain and discomfort. If untreated, serious long-term complications of the disease include critical limb ischemia and amputation in very extreme cases.
Types Of Vascular Stents
Each stent category can be further separated into Drug-Eluting Stents (DES) and Bare Metal Stents (BMS).
Bare metal stents were the original stents used to treat blocked vessels, which is simply a metal stent with no coating, whereas drug-eluting stents are coated with a drug which is slowly released into the surrounding tissue.
Drug-eluting stents are more effective than bare-metal stents at reducing restenosis (the blockage of vessels), however, concerns have been raised about the long term effect of drug-eluting stents. Some early generation drug-eluting stents have a higher risk of causing stent thrombosis after implantation.
Most drug-coated stents approved for peripheral vascular disease are coated with paclitaxel, a very potent cytotoxic agent. While the drug is effective are mitigating restenosis, the implications of the drug delaying normal tissue repair are not well understood.
With drug-eluting stents under review by the U.S. Food and Drug Administration (FDA), there is a growing interest in drug-free stent coatings that can mitigate restenosis.
Invisible Implantable Medical Devices
There is the option to move away from drug-coated stents toward biomaterials that avoid the normal reactions of the body caused by the presence of the stent.
A hydrophilic polymer coating that is bonded to the surface of a device such as a stent, while acting as an invisible barrier to the body’s defenses, could be the solution needed to tackle both thrombosis and restenosis.
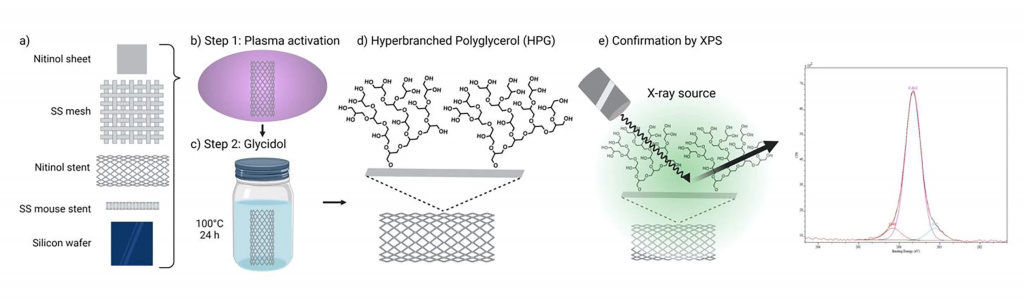
TekCyte has developed a nano-scale coating technology based on a hyperbranched polyglycerol (HPG) polymer (BIOINVISIBLE™) designed for stents and other endovascular devices to appear invisible to the human body.
This type of coated vascular medical device could be a vast improvement on existing vascular stents, with potential to improve the performance, safety and durability of short- and long-term implanted devices.
The new wave in vascular medical devices
The use of biomedical coatings to create better implantable devices is not restricted to vascular stents. These coating is being considered and applied to a variety of devices that are implantated into the body. TekCyte offers custom services that can integrate its BIOINVISIBLE™ coating with your implantable device. It can also investigate and design bespoke coatings that have tailored surface properties and functions, for your specific requirements.
Learn more about
Advances in biomedical equipment, systems and materials are opening new opportunities to create custom made biomedical coatings for the medical industry that have previously been unavailable or too expensive to consider developing for commercial applications.
Medical Device Product Development
"Our combined biological and biomaterials expertise means we can design ultra-thin, scalable coatings tailored to specific biomedical and medical needs and requirements," says Tony Simula, CEO at TekCyte.
Creating innovative medical devices that align with current regulations and best practices can be difficult. Custom made products can be time consuming and expensive to execute. With an individual and personalised design to achieve the desired outcome, tailor-made products can require extensive time and research to manufacture. However, with the use of biomedical coatings, medical device companies can alter the properties of their devices to suit their specific manufacturing and performance needs.
TekCyte is one medical manufacturing company that is leading the field in tailor-made coatings for a variety of applications to assist with an array of biomedical needs. Using the latest technologies, TekCyte can tailor-make coatings for medical devices and biomedical companies to create novel products for an increasingly competitive commercial landscape.
Custom Made Smart Health Devices
Our custom design advanced coatings that can significantly influence the behaviour and interaction of cells and living tissue with surfaces, enables us to develop any bioselective application. Once we know the nature of the desired cellular interaction with a natural or synthetic material, we can work with you to create a bioselective surface coating that performs as you would like it to. Our knowledge of the biomedical regulatory environment means that our processes are scalable and can meet international standards of quality and consistency.

Polymer coatings can be designed to enhance cell growth on the surface of many materials. This can be achieved by applying various polymer coatings alone or designing polymer coatings that allow the integration of cell-promoting biological molecules into the polymer coated surface.

Coatings can be created with specific chemical groups for rapid conjugation of biological molecules to a surface. Conjugated molecules can then be used for a range of biological applications, such as capturing target cells specifically on a treated surface.
Polymer coatings can be developed that encourage specific cells to adhere to surfaces that would not normally adhere. These surfaces can be used for extended periods (e.g. months) under culture conditions or submerged in aqueous environments.
When a very low background of non-specific binding is important; combine TekCyte’s BIOINVISIBLE™ coating with a cell capture molecule. Antibodies or aptamers can be conjugated directly onto our BIOINVISIBLE™ coating, to create a low binding surface that will only capture the cell of interest.

Coatings can be designed to manipulate the level of attachment of cells to a surface for specific conditions or environments.

Surface coatings can also be designed to reduce the adhesion of cells, proteins and/or microorganisms to the surface of a material, i.e. low-binding surfaces or low-fouling surfaces.
Custom Medical Device Contract Manufacturing
With its knowledge of medical manufacturing requirements, TekCyte takes a collaborative approach to partnering with medical device development companies to enhance their products. Working with national and international companies our technologies will allow the healthcare sector to offer better treatments and improved outcomes for its patients.
TekCyte has coating technologies with patents granted in Australia, United States, Europe, Japan and China, such as BIOINVISIBLE. It can create and test new biologically functional coatings for its customers or offer its own technologies, under license to create commercially-scalable custom made products. TekCyte offers this service on a project basis to suit the specific needs and requirements of the client company. This is a win-win situation for medical device and biomedical manufacturers.
This is a win-win situation for medical device manufacturers. TekCyte offers medical coating services to the market, with the expertise to create commercially-scalable custom made products
Learn more about
A non-pharmacological approach to reducing device-triggered thrombosis and restenosis
Synthetic materials are commonly rejected from the body’s natural defence mechanism. However, there is untapped potential to develop synthetic materials with the innate ability to avoid the body’s natural defence mechanisms from activating. While current materials used in devices has an acceptable level of biocompatibility, there are still resounding concerns leading to device failure. This is frequently occurring in the treatment of vessels for patients with peripheral arterial disease (PAD), where restenosis remains a significant challenge for vascular surgeons.
Eli Moore, Biomaterial Specialist at TekCyte, has recently co-authored Hyperbrancked polyglycerol coated vascular stents – a non-pharmacological approach to reducing device-triggered thrombosis and restenosis.
Explore the insights at ResearchSquare.com
By Dr Tony Simula, PhD MBA BSc(Hons), CEO at TekCyte
Peripheral arterial disease (PAD) is characterized by reduced blood flow in the arteries of the lower extremities, due to atherosclerotic occlusive disease. With over 200 million people now affected by the disease worldwide, it is the third leading cause of atherosclerotic vascular morbidity following stroke and coronary heart disease (Fowkes et al. 2013). There are several risk factors associated with the incidence of PAD including smoking, hypertension, diabetes, and obesity. The femoropopliteal region accounts for more than 60% of treated PAD cases, and symptoms can range from pain in the affected limb through to critical limb ischemia (CLI) with the risk of tissue necrosis and amputation.
Current treatments for PAD vary depending on the location and severity of the obstruction as well as the patient’s suitability for surgical intervention. Percutaneous vascular intervention (PVI) is usually favored over open surgery due to the reduced demand on the patient and the patients’ preference for minimally invasive techniques (Sanders et al. 2021). There are many PVI treatment options available, and currently there is no universally applicable treatment, as all currently approved, commercially available interventions suffer from a lack of long-term durability. The main cause of failure of these treatments is restenosis, due to intimal hyperplasia which is driven by an inflammatory response to the physical distension of the vessel wall and resultant damage caused by the intervention (Tan et al. 2021).
In an attempt to mitigate restenosis, the use of drug-eluting stents (DES) and drug-coated balloons (DCB) has increased significantly in recent years, with many studies demonstrating a significant improvement in patency compared with bare-metal stents (BMS) and plain old balloon angioplasty (POBA). However, longer-term follow up of PAD patients beyond 12 months has shown that their performance is no better than POBA or BMS (Tadwalkar, et al. 2015). Most drug-coated devices approved for the treatment of PAD are coated with paclitaxel, a very potent cytotoxic agent, but this potent cytotoxicity is proving to be a double-edged sword. The mechanism of action of the eluted cytotoxic drugs is to limit the growth of cells in the arterial wall. This not only inhibits the target cell growth but also delays normal tissue repair such as reendothelialization of the vessel wall or can cause local tissue necrosis. A trial comparing paclitaxel-coated balloons (PCB) with POBA had to be stopped due to an observed increase, from four per cent in POBA to eight per cent with PCB, in the number of amputations in the presence of the drug (Zeller et al 2014). A more recent systematic review and meta-analysis by Katsanos et al. (2018) reported a statistically significant increase in late all-cause mortality out to 5 years post-treatment, although no causal relationship was identified. This led the FDA to issue the first of several formal letters in January 2019 (FDA, 2019) to all health care providers, alerting them to the finding of increased all-cause mortality. In June 2019 the FDA convened a public meeting of the Circulatory System Devices Panel of the Medical Devices Advisory Committee and presented their own findings, which concurred with the original report by Katsanos et al. (2018).
More recent studies have failed to show a similar association with paclitaxel-coated devices, and though encouraging, they are limited by the duration of the follow-up, typically less than three years (Farb et al. 2021). This underscores the importance of the FDA’s recommendation to continue diligent long-term monitoring of patients who have been treated with paclitaxel-coated devices. In another study conducted in Germany using real-world data from over 37,000 patients, who underwent FP interventions over an eight-year period, it was demonstrated that patients treated with drug-coated devices had improved overall survival at five years when compared to a group of patients treated with POBA (Behrendt et al. 2020).
Many similar studies are underway and in time may provide a clearer understanding of the precise level of risk, a mechanism for the observed mortality signal, or identify specific populations where the risks are amplified. The debate still simmers as Katsanos and co. followed up their earlier 2018 article with another systematic review in 2021 suggesting an increased risk of amputations (from three to four per cent) in DCB-treated patients compared with POBA (Katsanos et al. 2021). Nonetheless, the FDA still recommends considering the use of drug-coated devices for patients at high risk of restenosis or repeat intervention.
Notwithstanding the recent controversy with paclitaxel, the high incidence of restenosis in PAD patients, compared with patients with coronary artery disease, is concerning and has been highlighted in several publications (Lee et al. 2016; Kokkinidis & Armstrong 2020; Tan et al. 2021). There are many perspectives on the reason for this difference. Factors might include the location, size and anatomy of the peripheral arteries compared with coronary and larger arteries. However, it appears clear that a different approach to the treatment of PAD is required to overcome the poor patency rates associated with the current paradigm.
With the established evidence for a role of inflammatory mediators such as cytokines and growth factors in the cause of restenosis (Libby et al. 2012, Maleknia et al. 2020; Tan et al. 2021), it has been proposed that a more targeted approach using immune-modulating biologicals/drugs instead of non-specific cytotoxic agents could more effectively reduce restenosis in PAD (Razavi et al. 2018; Maleknia et al. 2020; Tan et al. 2021). Razavi and co. showed in a human trial that the delivery of dexamethasone to the vessel adventitia, at the site of the intervention, was effective at reducing restenosis. A macrophage-directed approach has been proposed by Tan et al., suggesting that one or more cytokines such as TGF-b, IL-4 or IL-10, delivered locally at the site of stent insertion to treat a peripheral occlusion, may help to maintain a more anti-inflammatory (M2) phenotype in the recruited macrophage population, which would promote endothelial repair and reduce the likelihood of restenosis. While there may be great potential for the development of newer active-coated vascular devices, the task is a challenging one. The immune cells and the interplay of secreted factors at the site of vessel wall injury are very complex (Maleknia et al. 2020) and delivering the correct dosage of a factor equally demanding. Furthermore, the regulatory path becomes more arduous when a device is coated or combined with a biologically active molecule.
TekCyte has a novel coating technology that may provide a simple and unique solution to the problem of stent failure associated with the treatment of PAD. TekCyte’s coating – BIOINVISIBLE™ – is a highly hydrophilic polymer that is chemically bound to the surface of a device, providing a ‘protective’ barrier to the body’s normal defenses against foreign materials. BIOINVISIBLE™ is based on a hyperbranched polyglycerol that is ultra-thin ( less than 10nm), extremely stable and with no evidence of toxicity (Abbina et al. 2017; Jafari et al. 2020). The patented coating process is easily scalable and has recently been scaled up in TekCyte’s manufacturing facility in Adelaide, Australia.
As a hydrophilic coating, BIOINVISIBLE™ reduces the ability of proteins and cells to attach themselves to the surfaces of materials. In the case of blood-contacting devices, this can have profound effects on the binding and activation of platelets on the surface of products such as stents and stent-grafts. Platelets play a key role in the etiology of stent thrombosis, which is generally managed in patients with stents using dual antiplatelet therapy. In laboratory studies using human blood, BIOINVISIBLE™ can significantly reduce the binding and activation of platelets on the surface of a range of materials used in vascular devices.
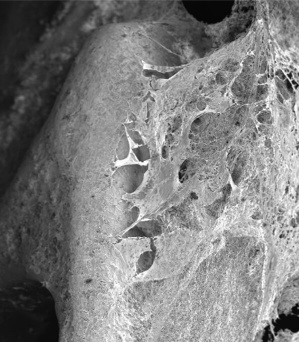
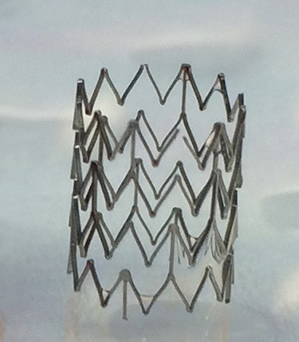
Extending the testing to clot formation, the development team has amassed considerable data showing the significant reduction in the level of thrombosis with coated stents using fresh human blood in the Chandler loop model. Images of the surface of nitinol stents and vascular grafts show dramatically reduced attachment of platelets to the surfaces, with a concomitant reduction in the formation of fibrin clots. Figure 1 below shows the obvious lack of thrombosis on a nitinol stent coated with BIOINVISIBLE™ compared with the equivalent bare metal stent. High power magnification of the stent struts confirms the lack of any fibrin clots on the coated stent.
The results in the Chandler loop have been verified using multiple blood donors and with various commercial stents. In the same studies, BIOINVISIBLE™ did not activate the complement cascade and in separate laboratory studies, BIOINVISIBLE™ showed no signs of cytotoxicity to blood cells and other cell types.
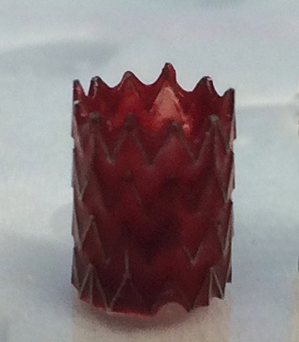
(clot formed in 30 min)
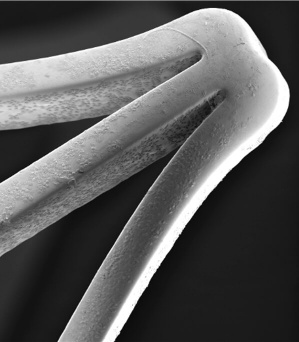
(no clot after 3 hours)
Figure 1: BIOINVISIBLE™ reduces clot formation in the Chandler loop
BIOINVISIBLE’s anti-thrombogenic properties have been demonstrated on several metallic substrates including nitinol, cobalt-chromium and stainless-steel stents. The coating process has also been extended to polymers used in vascular devices, including woven polyethylene terephthalate (PET) and expanded and woven polytetrafluoroethylene (PTFE, Teflon®), showing similar anti-thrombogenic results.
BIOINVISIBLE™ has great potential as a truly biocompatible coating for vascular devices. The very thin nature of the coating makes it suitable for coating the finest of device structures without interfering with their architecture and movement. The coating is also very robust and able to withstand the insertion of a coated stent into a stent delivery device. The coating’s anti-thrombogenic property is also unaffected when the same packaged nitinol stents are EO-sterilized and stored at room temperature for up to 3 years.
A study conducted in adult pigs showed that BIOINVISIBLE™ did not interfere with the normal regrowth of the vessel endothelium over the nitinol struts of coated stents placed in either the iliofemoral artery or iliofemoral vein. The image in Figure 2 shows the endothelium growing normally over the coated stent strut in the artery, within 5 days.
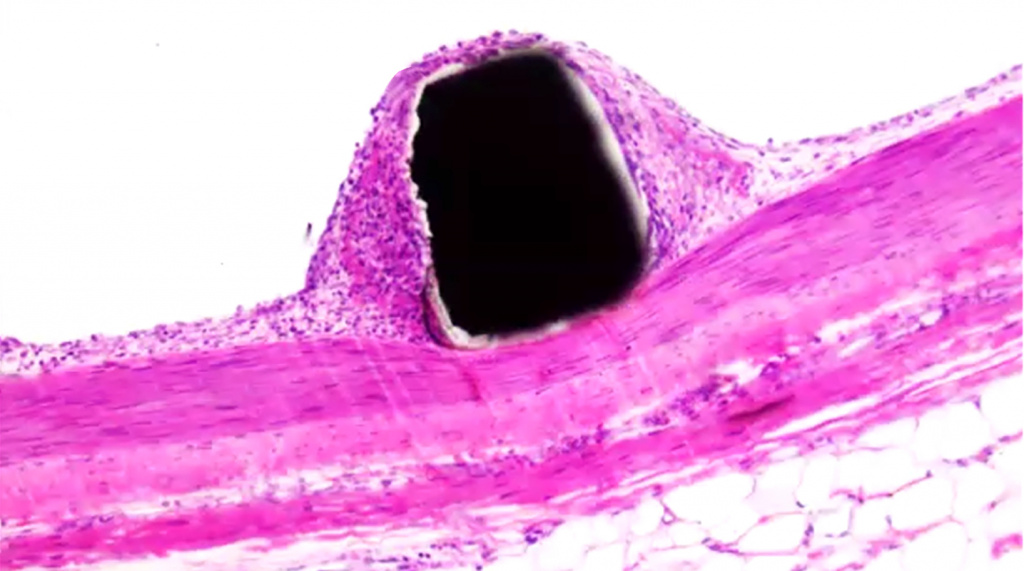
BIOINVISIBLE™ – coated nitinol stent strut
Endothelial lining
Smooth muscle
Figure 2: BIOINVISIBLE™ does not interfere with normal endothelial regrowth.
Using an established ApoE knock-out mouse model of restenosis (Ali et al. 2007; Vanags et al. 2018), BIOINVISIBLE™ dramatically reduced the level of restenosis in coated stainless-steel stents compared with uncoated stents. This result and the mechanism of action is the subject of ongoing investigations but strongly suggests that BIOINVISIBLE™ is an attractive and safe alternative medical coating to reduce both stent thrombosis as well as restenosis in stents used to treat PAD.
The advantage of TekCyte’s coating is the absence of a drug-eluting component, which removes the problem of tissue toxicity due to the eluted drug and any possibility of emboli from drug-coating particulates. This simple technology also allows for a rapid regulatory and manufacturing path to market.
TekCyte’s BIOINVISIBLE™ coating represents a disruptive medical advance, that has the potential to improve patency and durability of peripheral vascular therapies.
References:
- Fowkes FGR et al., Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010 – A systematic review and analysis. Lancet 2013; 382: 1329-1340.
- Sanders KM et al., Endovascular treatment of high-risk peripheral vascular occlusive lesions: A review of current evidence and emerging applications of intravascular lithotripsy, atherectomy, and paclitaxel-coated devices. Seminars in Vascular Surgery (2021); 34: 172-187.
- Tan RP et al., Macrophage Polarization as a novel therapeutic target for endovascular intervention in peripheral artery disease. JACC – Basic to Transl Sci 2021; 6(8): 693-704.
- Tadwalkar RV & Lee MS, The current state of endovascular intervention for peripheral artery disease. Vascular Disease Management 2015; 12(10): E190-E203.
- Zeller T, et al; Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol 2014; 64(15): 1568–1576.
- Katsanos K et al., Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: A systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2018;7(24).
- FDA, Paclitaxel-coated balloons and stents for peripheral arterial disease. FDA, 2019 (https://www.fda.gov/medical-devices/cardiovascular-devices/paclitaxel-coated-balloons-and-stents-peripheral-arterial-disease.
- Farb A et al., Drug-coated devices for peripheral arterial disease. N Engl J Med 2021; 384(2): 99-101.
- Behrendt C-A et al., Long term survival after femoropopliteal artery revascularisation with paclitaxel coated devices: a propensity score matched cohort analysis. Eur J Vasc Endovasc Surg 2020; 59: 587-596.
- Katsanos K & Spiliopoulos S, Safety of paclitaxel-eluting stents in below-the-knee arteries for critical limb ischemia treatment: The devil is in the details Cardiovasc Intervent Radiol 2020; 43: 1889–1890.
- Lee J-H et al., Development of a new hybrid biodegradable drug-eluting stent for the treatment of peripheral artery disease. BioMed Res Int 2016; 2016: 1-7.
- Kokkinidis DG & Armstrong EJ, Current developments in endovascular therapy of peripheral vascular disease. J Thorac Dis 2020; 12(4): 1681-1694.
- Libby et al., Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012; 32(9): 2045-2051.
- Maleknia M et al., Inflammatory growth factors and in-stent restenosis: Effect of cytokines and growth factors. SN Comprehensive Clinical Medicine 2020; 2: 397–407.
- Razavi MK et al., Adventitial drug delivery of dexamethasone to improve primary patency in the treatment of superficial femoral and popliteal artery disease. JACC Cardiovasc Interv 2018; 11(10): 921-931.
- Abbina S et al., Hyperbranched polyglycerols: recent advances in synthesis, biocompatibility and biomedical applications. J. Mater. Chem. B, 2017; 5: 9249-9277.
- Jafari M et al., Hyperbranched polyglycerol nanostructures for anti-biofouling, multifunctional drug delivery, bioimaging and theranostic applications. Int J Pharmaceut 2020; 576: 118959.
- Ali ZA et al., Increased in-stent stenosis in apoe knockout mice – Insights from a novel mouse model of balloon angioplasty and stenting. Arterioscler Thromb Vasc Biol. 2007; 27: 833-840.
- Vanags LZ et al., Apolipoprotein A-I reduces in-stent restenosis and platelet activation and alters neointimal cellular phenotype. JACC – Basic to Transl Sci 2018; 3(2): 200-209.
Every invention takes a team to bring it to market. It requires research and development, with a strong team carrying it through to the stage of commercialisation. The story behind BIOINVISIBLE™ is no different. With highly skilled researchers from a number of different scientific disciplines, a project team was created to work together to improve medical devices implanted in the body; BIOINVISIBLE™ was the result – a superior biocompatible coating for medical devices with the potential to render implanted devices ‘invisible’ to the body’s normal immune defences.
The world’s most advanced hyperbranched polyglycerol coating that can be applied at a commercial scale.
Current Stent Practises
The human body is well served by efficient defence mechanisms, which pose several biochemical challenges in the design of blood-contacting devices such as stents and vascular grafts, as well as non-vascular implants. Currently, medical device complications result in implant failure rates of up to 30 per cent, leaving many patients requiring additional medical intervention and surgery causing significant discomfort, morbidity or even death.
The standard practice for most stent manufacturers is to apply a dissolving layer containing a drug to bare-metal stents. These stents are called drug-eluting stents, which have been on the market for over 30 years. While this approach has improved patient outcomes it is known to lead to other complications down the line. These complications need to be managed and come at great personal costs to the patients and financial costs to the healthcare industry.
Professor Claudine Bonder (Centre for Cancer Biology, an Alliance between University of South Australia and SA Pathology) has a PhD in immunology with a particular interest in blood vasculature. She understands the importance of implanted devices to be invisible, however, this has eluded the medical device industry.
“If you were to lay out all the blood vessels in your body, they would reach over 100,000 kilometres,” says Claudine.
“Occupying such a large distance, research and development in vascular health are essential for long-term health and wellbeing of patients.”
With over 25,000 stents implanted into people each year in Australia each year and several million globally, a new concept for the next generation of stents was imperative.
The Research and Development
The initial funding that led to BIOINVISIBLE™ came via the Cooperative Research Centre for Cell Therapy Manufacturing (CTM CRC). The funded project was led by Claudine Bonder and included Professor Nico Voelcker (materials and nanotechnology engineer at the University of South Australia) to explore potential solutions to the issues around the body’s acceptance of a stent as a foreign object. The initial idea was to develop a coating that could act as an invisible cloak, stopping the defensive cells from attacking the stent, and to incorporate into the coating compounds that would help the damaged blood vessel around the stent to rapidly repair.
The first step was to make the device ‘invisible’. The addition of Dr Eli Moore to the team, a young PhD graduate focusing on the biological application of hyperbranched polyglycerol (HPG) polymers, was critical. Eli knew the potential of HPG coatings but realised that for such coatings to be commercially viable the existing approaches needed to be dramatically improved and the team was joined by Dr Glen Benveniste (vascular surgeon at Ashford Hospital).
“The traditional approach to hyperbranched polyglycerol polymers was to grow the polymer in a flask, then attach it to a surface, rather than to grow the polymer directly on the surface”, says Eli.
“I wanted to take this idea and expand it through my own research and connect with others to see what approaches would be feasible on a commercial scale.”
Eventually, a process for growing the HPG polymer directly on the surface of a stent was achieved. The process was novel, simple, environmentally friendly and easily scalable for commercial needs. Importantly, the coating was not only protective but reduced the formation of clots when applied to stents and tested in the laboratory. Two patents were lodged and have now been granted in the US and other major countries.
The second stage of the planned project was to change the HPG coating to pull in endothelial cells, naturally circulating in the blood, to the location of the stent. This would help making the stent safe. But as the new HPG coating was so effective at reducing clots, this second was not required. Focusing on a simple coating sped up development of HPG, and the final product would also reduce manufacturing costs, making it more attractive to medical device companies, and the patients.
Merging with TekCyte
Tony Simula, CEO of TekCyte, was Program Manager of the original CTM CRC project and played a vital role in explaining to the CRC Board that HPG had tremendous commercial potential and getting further funding to develop the technology. This was made easier given the commercial interest the new coating was already receiving from global device manufacturers. Their involvement in the early phase helped the team remain commercially focused and gave them an insight into the path to market for the coating technology.
“The idea of using HPG had significant possibilities to transform the current medical device coating landscape, and I didn’t want to leave the concept without exploring it fully,” says Tony.
“The research showed the ability to address the failure of implantable devices, particularly vascular stents, that can lead to blood clots and other complications.”
“When the opportunity came to provide medical device manufacturers with an alternative stent coating, it was impossible to say no.”
Driven by international medical device company interest, it was decided to continue the development of the coating technology and in-license the patent rights of the new coating into the newly formed company, TekCyte. The ‘BIOINVISIBLE’ name was soon applied to the patented HPG coating process. Tony remains the company’s CEO, which recently completed a round of capital raising to support the commercialisation of BIOINVISIBLE™. With the additional funds, TekCyte is now exploring new markets opportunities for BIOINVISIBLE™, and its unique properties, for industrial applications.
Despite BIOINVISIBLE™ being in the early stages, it is ready for clinical and commercial-scale manufacture at TekCyte’s Mawson Lakes facility. The BIOINVISIBLE™ story is yet to be finished, but it has moved into the next chapter.
Learn how it works
Our bodies are fascinating machines; the way they can repair themselves after injury, or become immune after infection. However even the most advanced machines have flaws. Due to our bodies’ impressive ability to protect against diseases and foreign objects, the rise in biomaterial, and in particular implanted medical devices, surgeons and patients continue to risk complications. At the same time device manufacturers are continually faced with the challenge of reducing complications by creating safer devices.
Biomaterials play a significant and vital role in modern medicine and help with the restoration and healing of patients after a disease, injury, or chronic disorder. However, with the use of certain artificial biomaterials, our bodies react in the only way it knows how, to attack the foreign object. All implanted materials are recognised by a patient’s immune system as foreign, causing cellular and tissue immune responses. If the body responds negatively to the implant, it can lead to inflammation, slowing the healing process, and even failure of the implant. In most cases the body positively responds to an implant, leading to successful biointegration. Hence, the benefits of medical devices far outweigh the risks, allowing for their continued use in today’s medical practice.
The Opportunity
The medical device industry is always seeking to enhance the current devices and improving how the body will react to them. However with so many implants on the market, it is not feasible to redesign all the products to improve their longevity in the body. Fortunately for surgeons and medical practitioners, TekCyte has spent significant time researching exactly this issue to bridge the gap between already effective products, yet improve their likelihood to succeed within the body. For example, over the last several years, TekCyte’s team and its collaborators have developed a patented coating (BIOINVISIBLE™) enabling some devices to appear invisible to the body’s defences, potentially improving the safety and extending the life of implanted devices. The potential for a universal biocompatible coating that will improve all devices is vast, but as we gain a better understanding of how our body responds to foreign material, we are seeking more targeted approaches to making better performing and safer devices.
The Coatings
Device complications may vary for different devices and their location in the body, requiring unique ways to address the issues. There are multiple options one can take when choosing the right coating for a medical device or biomedical application.
TekCyte’s expertise is built around the understanding of and ability to manipulate material surface properties and their interaction with living cells and tissue. The list below provides examples of other advanced coatings with particular biological functions that can be designed and developed by TekCyte for specific purposes. These applications extend beyond implantable devices to include medical research and discovery, diagnostics and bioprocessing.

Cell Growth
Our coatings can be designed to enhance cell growth on the surface of materials through the integration of cell-promoting biological molecules onto the surface of the product.
Cell Adhesion
Similar to cell attachment, coatings can be designed to manipulate the degree of adhesion a cell will exhibit toward the surface of a material. This is useful when transferring cells from a coated device to a location on the body, such as a wound or when the interaction of cells with a surface needs to be transient.
Cell Capture
Coatings can be created to allow for capturing targeted cells on a treated surface. Through utilising specific chemical groups for rapid conjugation of molecules, the coating can allow for chosen cells to stick to the surface.
Cell Repelling
It’s also possible to achieve the opposite effect to cell attachment/adhesion. Coatings, such as BIOINVISIBLE™, totally inhibit the adhesion of cells, and potentially proteins and/or microorganisms to the surface of a product, allowing for an easier removal of the product or to avoid detection by the body’s immune cells. Ultimately, making the product ‘invisible’ to the body.
Cell Attachment
Our coatings can be tailored to make certain cells attach to a surface that they would naturally not attach themselves to. For example, by encouraging the attachment of specific cells, research and discovery of new treatments for disease can be dramatically enhanced.
Cell Selective
Through the use of TekCyte’s BIOINVISIBLE™ coating, and with the addition of a targeted cell capture molecule (e.g., antibody, aptamer), we can create an invisible barrier with virtually no background binding of cells to the surface, apart from the cell of interest.
TekCyte’s team of biomedical researchers and materials engineers can work closely with device manufacturers and biomedical product suppliers to develop advanced coated devices and biomedical products, helping our partners to become leaders in their fields.
CYPATCH is a new contender in active wound dressings, pairing the Cymerus® cell technology to tackle major challenges in wound care, including diabetic foot ulcers (DFU). TekCyte’s CYPATCH is a proprietary surface-coating for wound dressings, optimised to deliver adult mesenchymal stem cells directly to the wound bed, accelerating the healing process. This coating technology has been exclusively licensed to Cynata Therapeutics Limited (ASX: CYP or “Cynata”).
For many patients with diabetes, wounds do not heal easily due to reduced blood flow, especially in the legs and feet. These wounds then become easily infected further delaying the healing process. With over 34 per cent of diabetic patients developing these chronic wounds, there are over half a million patients in Australia suffering from ulcers, with most occurring on their feet. If these wounds are not treated, they can lead to life threatening sepsis and/or amputation. CYPATCH is a patented coating technology developed to heal difficult wounds like DFU, by delivering stem cells to the wound, that produce a cocktail of factors to kickstart the healing process. The coating is designed to allow live cells to separate from the dressing and enter the wound, aiding with rejuvenation and the healing process, which is extremely beneficial for non-healing wounds like DFU.
For medical product manufacturing, there is a long process and timeframe from the original research to commercial product, and the story behind CYPATCH is no different. In 2013, the initial project was funded by the Cooperative Research Centre for Cell Therapy Manufacturing (CTM CRC) and led by Dr Louise Smith, former Lecturer in Advanced Materials and Research Fellow at University of South Australia. The project co-existed with a sister project, led by Professor Alison Cowin, Professor of Regenerative Medicine at the University of South Australia, that assisted with the pre-clinical testing of the wound dressing technology. The initial team was rounded off with Prof Rob Short, Post-Doctoral Researchers, Dr Giles Kirby and Dr Stuart Mills, and Plasma Physicist Dr Andrew Michelmore, all of whom are inventors on the patent.
The key to the dressing was finding the ‘sweet spot’ where the cells attached to the dressing but not too strongly that they wouldn’t come off again and enter the wound. After this initial research, the team had the building blocks needed to bring the research to clinical testing for the treatment of DFU’s, however, it required some fine tuning. Once this was successfully completed the pre-clinical testing of the new dressing in both normal and diabetic mouse models were undertaken and showed the technology had potential as a new active wound dressing.

Merging with TekCyte
With a research team who believed in the potential of the product, and armed with positive animal model data, the project was assigned to TekCyte, bringing together the original lead researchers with new medical researchers to generate commercial interest in the technology.
In 2017, a collaborative proof-of-concept study was conducted by TekCyte and Cynata, supported by CTM CRC, using Cynata’s Cymerus® cells, to see if CYPATCH would work to deliver their cells. In preclinical studies, comparing the rate of wound healing, the combination of CYPATCH plus Cymerus® cells outperformed other CYPATCH + cell combinations. With the research underway and a solid product ready for more extensive testing, the team at TekCyte and Cynata set a course to establish the manufacturing process for the combination product that would be suitable for human clinical trials.
The Clinical Trials
In 2021 Cynata licensed the CYPATCH technology from TekCyte with the aim of conducting a first-in-man trial of CYPATCH with Cymerus® cells. In December 2021, Cynata announced the commencement of the clinical trial, which aims to treat 30 adult patients with DFU, comparing CYPATCH populated with Cynata’s Cymerus® cells (CYP-006TK) with the standard care. The trial will focus on two primary outcomes. Firstly the safety of the application, and secondly the rate of healing of the wound, together with monitoring pain and quality of life at 12 and 24 weeks after treatment.
The trial will take place at Royal Adelaide Hospital and The Queen Elizabeth Hospital, Adelaide. The clinical trial will be overseen by Professor Robert Fitridge, Professor of Vascular Surgery at the University of Adelaide, and Consultant Vascular Surgeon with the Central Adelaide Local Health Network. Cynata plans to complete the trial in 2022.
With a strong potential for improving the lives of many diabetic patients suffering from DFU, the CYPATCH coating delivering Cymerus® cells is on track to becoming a game changer in the advanced wound care industry.
Learn how it works
TekCyte is a world leader in the development and manufacture of advanced coatings for medical devices and biomedical applications.
Contact Us
Company: Tekcyte
Address: Level 3, Material and Minerals (MM) Building, UniSA Mawson Lakes Campus, Mawson Lakes, SA, 5095, Australia
Phone: +61 8 8302 3491
Email: info@tekcyte.com